
Mediterranean Sea Ban Algebra periodic table ocr a level Sober Nuclear
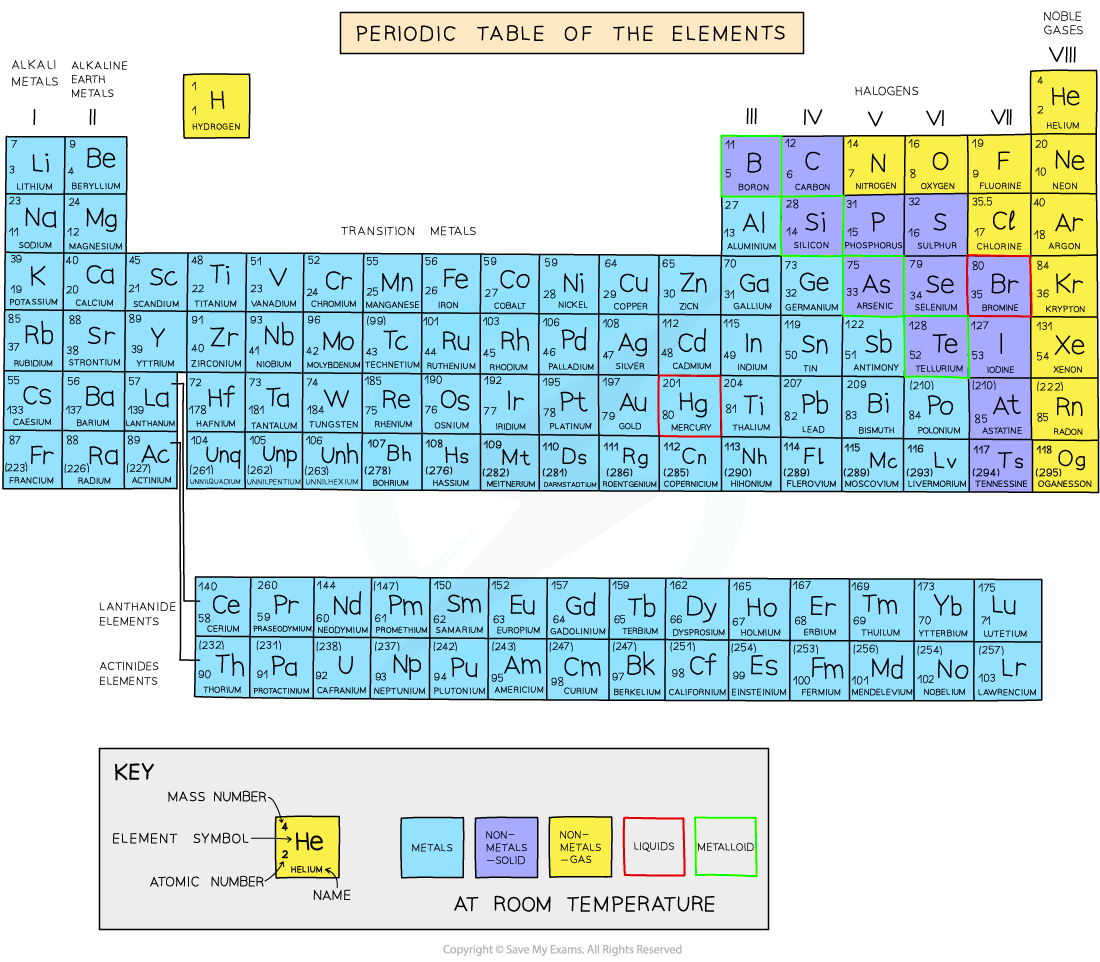
The Periodic Table The Periodic Table of the Elements Exam Tip The atomic number is unique to each element and could be considered as an element's "fingerprint". The number of electrons changes during chemical reactions, but the atomic number does not change. You've read 1 of your 10 free revision notes Get unlimited access
Atoms & Bonds Middle School Science
Interactive Periodic Table with lots of information available, from melting and boiling points to discovery and uses. Discovery dates. A simple interactive Periodic Table which enables you to track the discovery dates of the elements and relate them to things like reactivity and percentage abundance in the Earth's crust. Periodic Table videos.

IGCSE Chemistry Periodic Table UCLES Free Download, Borrow, and
4 IGCSE CHEMISTRY STUDY NOTES UNIT 3 ATOMS, ELEMENTS & COMPOUNDS C Key for using Periodic Table NOTE: A different format may be used in some questions. In any case, the greater number is always the Atomic mass number and the smaller number is the Atomic number. Exception: 𝟏 𝟏, where Z = 1 & A = 1
Chemistry IGCSE Classroom
1 The number of protons and neutrons is the same. 2 The number of protons and electrons is the same. 3 The number of outer electrons is one. 25 The diagrams show the electron arrangements in the atoms of four elements. 26 The atomic structures of four atoms are shown.

cambridge igcse study help the periodic table and electronic structure
1 1 mark The structure of four particles is described in the table. What are the correct values for X, Y and Z? Choose your answer Stuck? View related notes Did this page help you? FREE Chemistry revision notes on Kinetic Theory. Designed by the teachers at SAVE MY EXAMS for the CIE IGCSE Chemistry 0620 / 0971 syllabus.
[最も選択された] gcse periodic table igcse 255742 Gambarsaevit
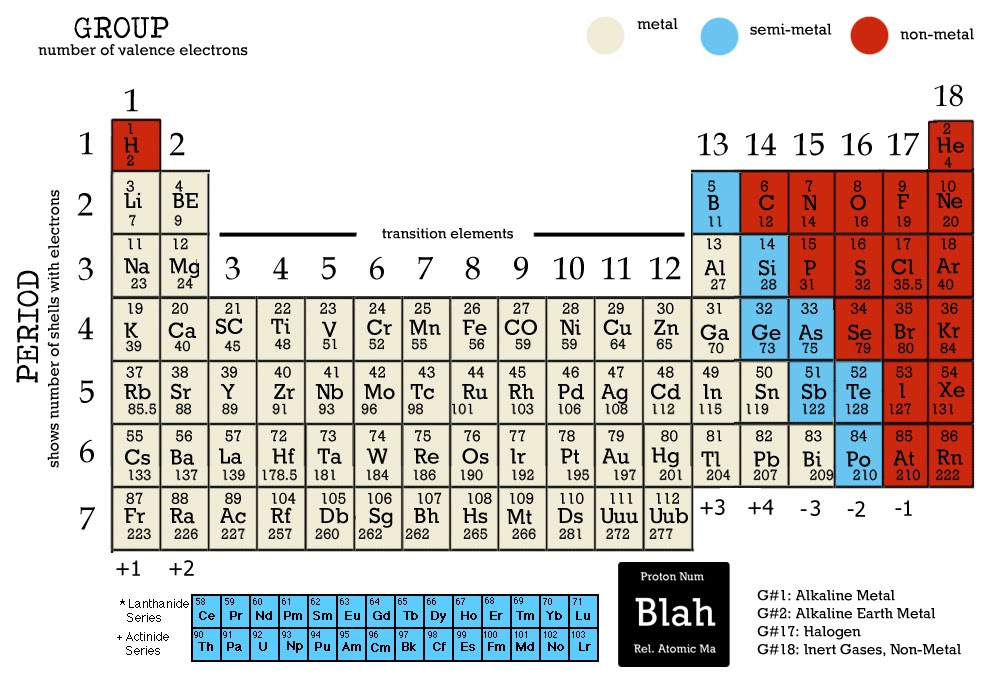
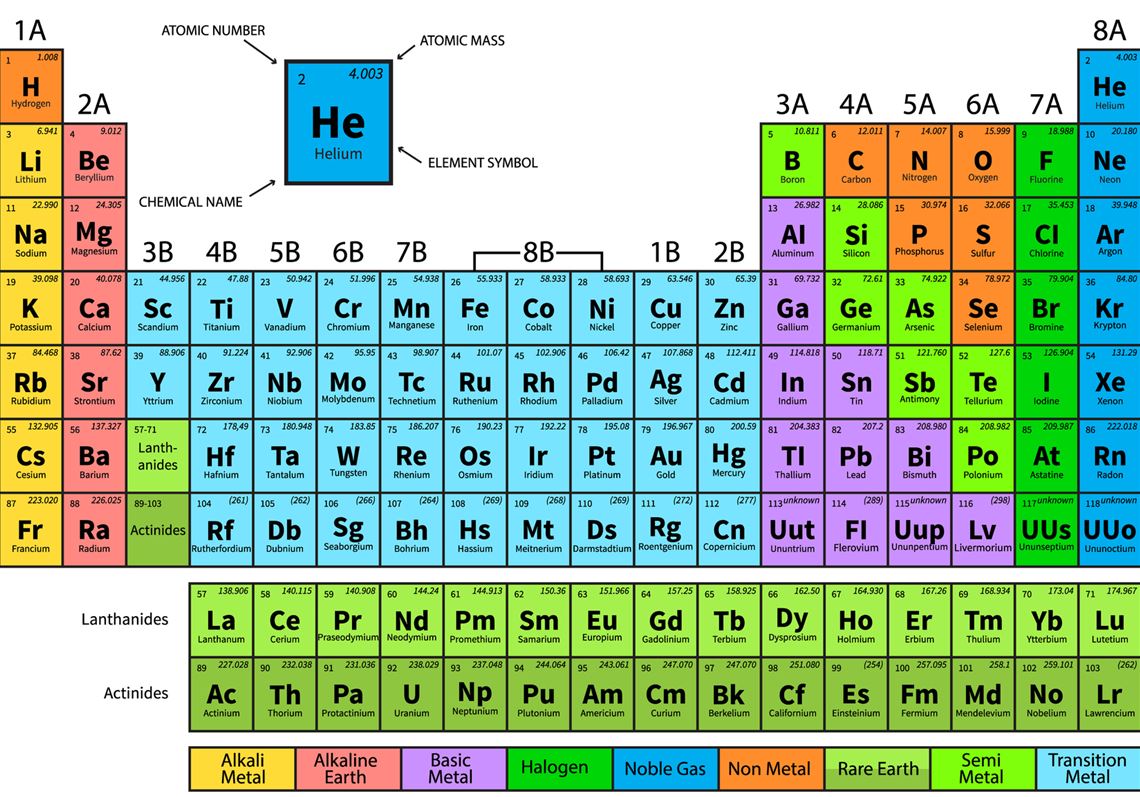
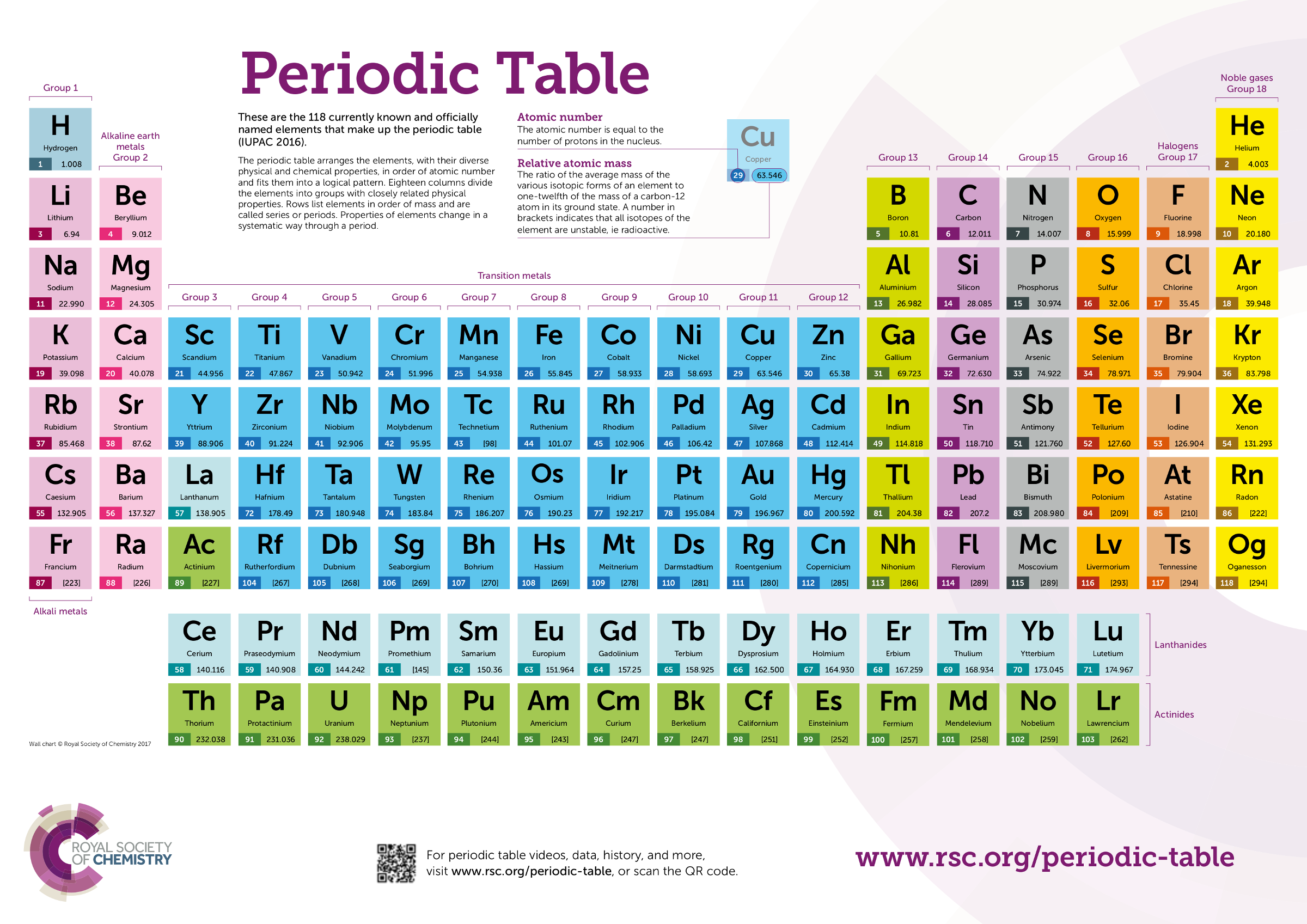
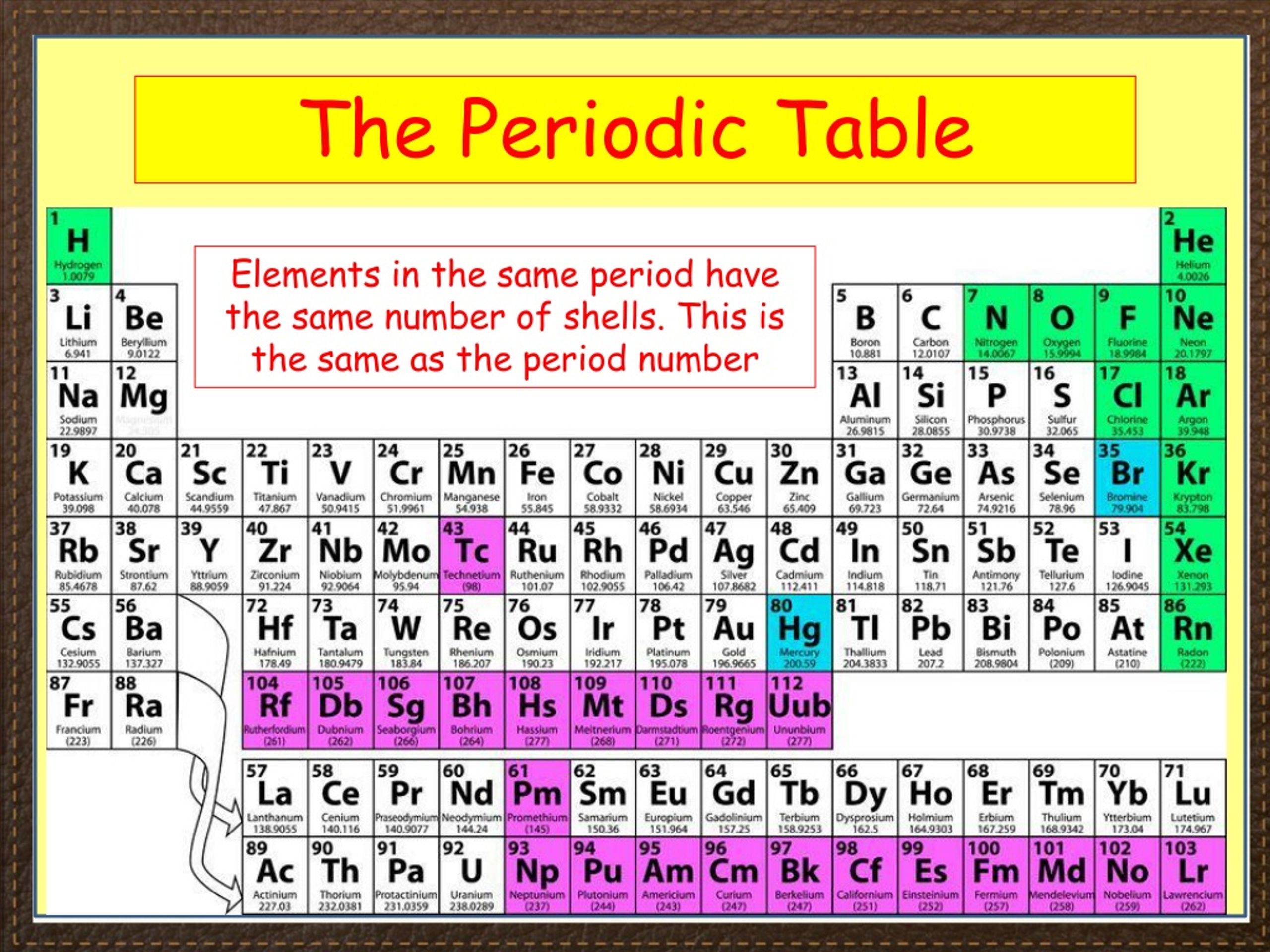
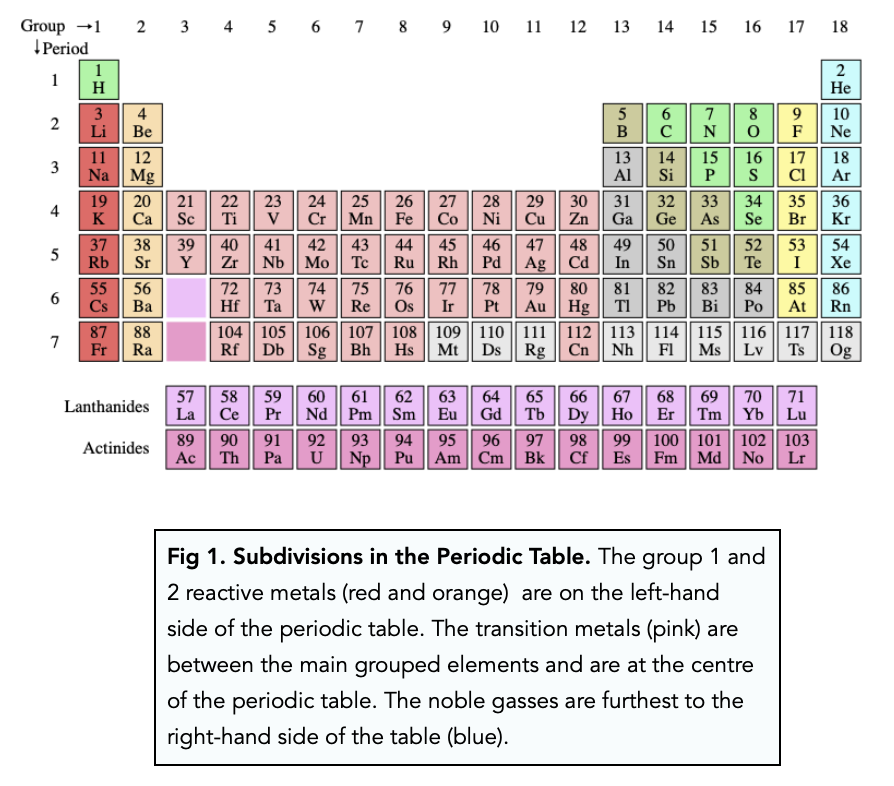
Period: These are the horizontal rows that show the number of shells of electrons an atom has and are numbered from 1 - 7 E.g. elements in period 2 have two electron shells, elements in period 3 have three electron shells
IGCSE Chemistry Notes Chapter 3 The Periodic Table
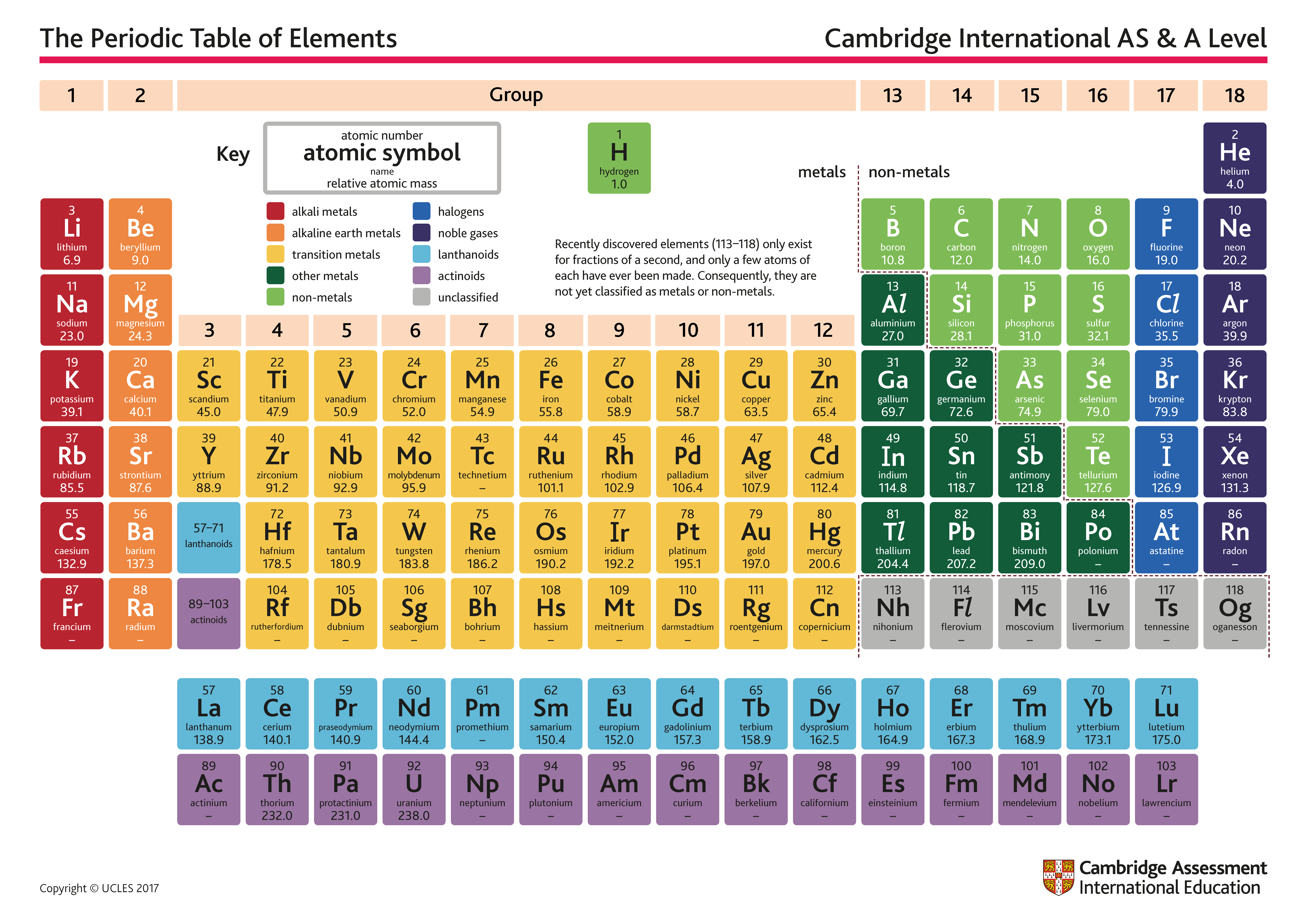
The Periodic Table. Section 1: Understanding The Periodic Table. The periodic table is a table of chemical elements arranged in order of their relative atomic numbers.. The vertical columns are called 'groups' and the horizontal rows are called 'periods'.. Elements in the same group have the same number of electrons in their outermost shell and hence, have similar chemical properties.

SOLUTION iGCSE Chemistry Periodic Table Studypool
The periodic table which also used to be known as the mendeleev table lists down all the elements which have been discovered till now. In the modern periodic table, the vertical columns are known as groups whereas the horizontal rows are known as periods. The elements are arranged in order of increasing atomic number.
PPT iGCSE chemistry Section 2 lesson 1 PowerPoint Presentation, free
2.2 recall the positions of metals and non-metals in the Periodic Table. From left to right across a period there is a gradual change from metal to non-metal elements. For example, in Period 3, sodium, magnesium and aluminium are metals. They all conduct electricity and their oxides are basic.

SOLUTION Cambridge IGCSE chemistry periodic table Studypool
An atom is the smallest uncharged particle that can take part in a chemical change. An atom contains a centrally located nucleus. The nucleus contains positively charged protons and neutral neutrons. [Protons + neutrons= nucleon number or mass number] The electrons revolve around the nucleus in fixed orbits called electron shells or energy levels.

igcse chemistry 113 understand that the periodic table color periodic
What is the Periodic Table? You met the Periodic Table briefly in Chapter 3. Let's review its key points. The Periodic Table is a way of classifying the elements. tell you about the particles in the It shows them in order of their proton number. Lithium has 3 protons, beryllium has 4, boron has 5, and so on.
Edexcel IGCSE Chemistry 复习笔记 1.4.1 Periodic Table Basics翰林国际教育
The Periodic Table 34 Safety in the laboratory 35 Mathematical requirements 35 Presentation of data 36 ICT opportunities 37 Conventions (e.g. signs, symbols, terminology and nomenclature) 37. 'Cambridge IGCSE is one of the most sought-after and recognised qualifications in the world. It
9701_RP_Planning Online Learning area
Cambridge IGCSE Chemistry Topic 9: The Periodic Table The Periodic Table Notes www.pmt.education. Describe the Periodic Table as a method of classifying elements and its use to predict properties of elements The Periodic Table can be used to classify elements and predict properties of.

cambridge igcse study help the periodic table and electronic structure
Kick-start your revision with our 2-day online Mock Preparation courses. Suitable for separate and combined science higher level students. Science AQA GCSE and Edexcel IGCSE - 2-3rd and 5-6th January. Book your place now! This topic is included in Paper 1, Paper 2, Paper 3, Paper 4, Paper 5 and Paper 6 for IGCSE CAIE Chemistry.
Periodic Table Metals vs NonMetals (GCSE Chemistry) Study Mind
Periodic Trends & Electronic Configuration The electronic configuration is the arrangement of electrons into shells for an atom (e.g: the electronic configuration of carbon is 2,4) There is a link between the electronic configuration of the elements and their position on the Periodic Table
Periodic Table Trends Paper 2 Solved MCQs IGCSE Chemistry 0620/ O Level
Download PDF Test Yourself Classifying Elements & Predicting Properties Periodic table Elements are arranged on the Periodic Table in order of increasing atomic number, where each element has one proton more than the element preceding it